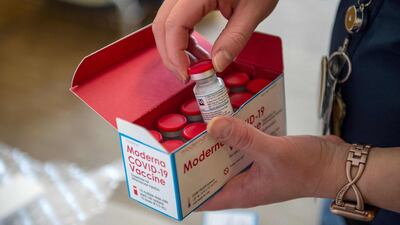
The EU’s drugs watchdog held off authorising Moderna’s coronavirus jab on Monday despite bringing forward a special meeting, as criticism mounts of the bloc’s slow launch of vaccines.
The Amsterdam-based European Medicines Agency said it would resume talks on Wednesday on whether to give the green light to the drug.
Under pressure from member states to speed up, the regulator fast-tracked the meeting to decide on approval from January 12 to Wednesday, then again to Monday.
Despite launching its immunisation campaign with the Pfizer-BioNTech vaccine, on December 27, the EU’s progress has been far slower than that of in the US, Britain and Israel.
“EMA’s committee for human medicines discussion on Covid-19 vaccine [by] Moderna has not concluded today. It will continue on Wednesday,” the EMA said on Twitter.
“No further communication will be issued today by EMA.”
The European Commission had earlier defended the bloc against criticism, and said its plans would get the EU past “bumps on the road”.
“It’s obvious that such a complex endeavour is always going to bring with it difficulties,” spokesman Eric Mamer told journalists.
The Pfizer-BioNTech vaccine, which was developed in Germany, is the only one currently authorised for use in the EU since its fast-track authorisation by the EMA on December 21.
America uses it alongside the Moderna vaccine, while the UK as of Monday also started using one by Oxford University and British pharma company AstraZeneca.
EU states have lagged far behind. France, for instance, has given only about 500 people their first jabs. Germany has started to immunise about 200,000.
The Netherlands, the last member state to start vaccinations, said it would bring the start of jabs forward by two days, to Wednesday.
The European Commission emphasised it had bought access to almost two billion doses of six potential vaccines, enough to immunise the EU’s entire population twice.
US-based Moderna's jab was found to be 94.1 per cent effective in preventing Covid-19 compared with a placebo in a clinical trial of 30,000 people, performing slightly better in younger adults than in the elderly.
The EMA said last week the coronavirus vaccine developed by AstraZeneca and Oxford University, which was approved Wednesday in Britain, is unlikely to get a green light in the EU in the next month.
That the watchdog moved from London to Amsterdam after Brexit has itself fuelled commentary about how Britain had been able to move faster after leaving the EU.
Saturday's results
Brighton 1-1 Leicester City
Everton 1-0 Cardiff City
Manchester United 0-0 Crystal Palace
Watford 0-3 Liverpool
West Ham United 0-4 Manchester City
KEY%20DATES%20IN%20AMAZON'S%20HISTORY
Empty Words
By Mario Levrero
(Coffee House Press)
Abu Dhabi traffic facts
Drivers in Abu Dhabi spend 10 per cent longer in congested conditions than they would on a free-flowing road
The highest volume of traffic on the roads is found between 7am and 8am on a Sunday.
Travelling before 7am on a Sunday could save up to four hours per year on a 30-minute commute.
The day was the least congestion in Abu Dhabi in 2019 was Tuesday, August 13.
The highest levels of traffic were found on Sunday, November 10.
Drivers in Abu Dhabi lost 41 hours spent in traffic jams in rush hour during 2019
AI traffic lights to ease congestion at seven points to Sheikh Zayed bin Sultan Street
The seven points are:
Shakhbout bin Sultan Street
Dhafeer Street
Hadbat Al Ghubainah Street (outbound)
Salama bint Butti Street
Al Dhafra Street
Rabdan Street
Umm Yifina Street exit (inbound)
MATCH INFO
Barcelona 4 (Suarez 27', Vidal 32', Dembele 35', Messi 78')
Sevilla 0
Red cards: Ronald Araujo, Ousmane Dembele (Barcelona)
Terror attacks in Paris, November 13, 2015
- At 9.16pm, three suicide attackers killed one person outside the Atade de France during a foootball match between France and Germany
- At 9.25pm, three attackers opened fire on restaurants and cafes over 20 minutes, killing 39 people
- Shortly after 9.40pm, three other attackers launched a three-hour raid on the Bataclan, in which 1,500 people had gathered to watch a rock concert. In total, 90 people were killed
- Salah Abdeslam, the only survivor of the terrorists, did not directly participate in the attacks, thought to be due to a technical glitch in his suicide vest
- He fled to Belgium and was involved in attacks on Brussels in March 2016. He is serving a life sentence in France
If you go
The Flights
Emirates and Etihad fly direct to Johannesburg from Dubai and Abu Dhabi respectively. Economy return tickets cost from Dh2,650, including taxes.
The trip
Worldwide Motorhoming Holidays (worldwidemotorhomingholidays.co.uk) operates fly-drive motorhome holidays in eight destinations, including South Africa. Its 14-day Kruger and the Battlefields itinerary starts from Dh17,500, including campgrounds, excursions, unit hire and flights. Bobo Campers has a range of RVs for hire, including the 4-berth Discoverer 4 from Dh600 per day.
10 tips for entry-level job seekers
- Have an up-to-date, professional LinkedIn profile. If you don’t have a LinkedIn account, set one up today. Avoid poor-quality profile pictures with distracting backgrounds. Include a professional summary and begin to grow your network.
- Keep track of the job trends in your sector through the news. Apply for job alerts at your dream organisations and the types of jobs you want – LinkedIn uses AI to share similar relevant jobs based on your selections.
- Double check that you’ve highlighted relevant skills on your resume and LinkedIn profile.
- For most entry-level jobs, your resume will first be filtered by an applicant tracking system for keywords. Look closely at the description of the job you are applying for and mirror the language as much as possible (while being honest and accurate about your skills and experience).
- Keep your CV professional and in a simple format – make sure you tailor your cover letter and application to the company and role.
- Go online and look for details on job specifications for your target position. Make a list of skills required and set yourself some learning goals to tick off all the necessary skills one by one.
- Don’t be afraid to reach outside your immediate friends and family to other acquaintances and let them know you are looking for new opportunities.
- Make sure you’ve set your LinkedIn profile to signal that you are “open to opportunities”. Also be sure to use LinkedIn to search for people who are still actively hiring by searching for those that have the headline “I’m hiring” or “We’re hiring” in their profile.
- Prepare for online interviews using mock interview tools. Even before landing interviews, it can be useful to start practising.
- Be professional and patient. Always be professional with whoever you are interacting with throughout your search process, this will be remembered. You need to be patient, dedicated and not give up on your search. Candidates need to make sure they are following up appropriately for roles they have applied.
Arda Atalay, head of Mena private sector at LinkedIn Talent Solutions, Rudy Bier, managing partner of Kinetic Business Solutions and Ben Kinerman Daltrey, co-founder of KinFitz
Company profile
Name: Fruitful Day
Founders: Marie-Christine Luijckx, Lyla Dalal AlRawi, Lindsey Fournie
Based: Dubai, UAE
Founded: 2015
Number of employees: 30
Sector: F&B
Funding so far: Dh3 million
Future funding plans: None at present
Future markets: Saudi Arabia, potentially Kuwait and other GCC countries
Read more
Company Fact Box
Company name/date started: Abwaab Technologies / September 2019
Founders: Hamdi Tabbaa, co-founder and CEO. Hussein Alsarabi, co-founder and CTO
Based: Amman, Jordan
Sector: Education Technology
Size (employees/revenue): Total team size: 65. Full-time employees: 25. Revenue undisclosed
Stage: early-stage startup
Investors: Adam Tech Ventures, Endure Capital, Equitrust, the World Bank-backed Innovative Startups SMEs Fund, a London investment fund, a number of former and current executives from Uber and Netflix, among others.
ETFs explained
Exhchange traded funds are bought and sold like shares, but operate as index-tracking funds, passively following their chosen indices, such as the S&P 500, FTSE 100 and the FTSE All World, plus a vast range of smaller exchanges and commodities, such as gold, silver, copper sugar, coffee and oil.
ETFs have zero upfront fees and annual charges as low as 0.07 per cent a year, which means you get to keep more of your returns, as actively managed funds can charge as much as 1.5 per cent a year.
There are thousands to choose from, with the five biggest providers BlackRock’s iShares range, Vanguard, State Street Global Advisors SPDR ETFs, Deutsche Bank AWM X-trackers and Invesco PowerShares.
Trolls World Tour
Directed by: Walt Dohrn, David Smith
Starring: Anna Kendrick, Justin Timberlake
Rating: 4 stars
Six large-scale objects on show
- Concrete wall and windows from the now demolished Robin Hood Gardens housing estate in Poplar
- The 17th Century Agra Colonnade, from the bathhouse of the fort of Agra in India
- A stagecloth for The Ballet Russes that is 10m high – the largest Picasso in the world
- Frank Lloyd Wright’s 1930s Kaufmann Office
- A full-scale Frankfurt Kitchen designed by Margarete Schütte-Lihotzky, which transformed kitchen design in the 20th century
- Torrijos Palace dome
The finalists
Player of the Century, 2001-2020: Cristiano Ronaldo (Juventus), Lionel Messi (Barcelona), Mohamed Salah (Liverpool), Ronaldinho
Coach of the Century, 2001-2020: Pep Guardiola (Manchester City), Jose Mourinho (Tottenham Hotspur), Zinedine Zidane (Real Madrid), Sir Alex Ferguson
Club of the Century, 2001-2020: Al Ahly (Egypt), Bayern Munich (Germany), Barcelona (Spain), Real Madrid (Spain)
Player of the Year: Cristiano Ronaldo, Lionel Messi, Robert Lewandowski (Bayern Munich)
Club of the Year: Bayern Munich, Liverpool, Real Madrid
Coach of the Year: Gian Piero Gasperini (Atalanta), Hans-Dieter Flick (Bayern Munich), Jurgen Klopp (Liverpool)
Agent of the Century, 2001-2020: Giovanni Branchini, Jorge Mendes, Mino Raiola
Specs
Engine: 2-litre
Transmission: Eight-speed automatic
Power: 255hp
Torque: 273Nm
Price: Dh240,000
COMPANY%20PROFILE
Herc's Adventures
Developer: Big Ape Productions
Publisher: LucasArts
Console: PlayStation 1 & 5, Sega Saturn
Rating: 4/5
Turkish Ladies
Various artists, Sony Music Turkey
Neighbourhood Watch
Day 2, Dubai Test: At a glance
Moment of the day Pakistan’s effort in the field had hints of shambles about it. The wheels were officially off when Wahab Riaz lost his run up and aborted the delivery four times in a row. He re-measured his run, jogged in for two practice goes. Then, when he was finally ready to go, he bailed out again. It was a total cringefest.
Stat of the day – 139.5 Yasir Shah has bowled 139.5 overs in three innings so far in this Test series. Judged by his returns, the workload has not withered him. He has 14 wickets so far, and became history’s first spinner to take five-wickets in an innings in five consecutive Tests. Not bad for someone whose fitness was in question before the series.
The verdict Stranger things have happened, but it is going to take something extraordinary for Pakistan to keep their undefeated record in Test series in the UAE in tact from this position. At least Shan Masood and Sami Aslam have made a positive start to the salvage effort.
While you're here
Con Coughlin: To survive, Nato must renew its sense of common purpose
Gavin Esler: Nato summit failed for making news more than it made deals
Simon Waldman: Nato continues to be Ankara’s best security guarantor
MATCH INFO
What: 2006 World Cup quarter-final
When: July 1
Where: Gelsenkirchen Stadium, Gelsenkirchen, Germany
Result:
England 0 Portugal 0
(Portugal win 3-1 on penalties)
Company profile
Name: Oulo.com
Founder: Kamal Nazha
Based: Dubai
Founded: 2020
Number of employees: 5
Sector: Technology
Funding: $450,000
What can victims do?
Always use only regulated platforms
Stop all transactions and communication on suspicion
Save all evidence (screenshots, chat logs, transaction IDs)
Report to local authorities
Warn others to prevent further harm
Courtesy: Crystal Intelligence
Know your camel milk:
Flavour: Similar to goat’s milk, although less pungent. Vaguely sweet with a subtle, salty aftertaste.
Texture: Smooth and creamy, with a slightly thinner consistency than cow’s milk.
Use it: In your morning coffee, to add flavour to homemade ice cream and milk-heavy desserts, smoothies, spiced camel-milk hot chocolate.
Goes well with: chocolate and caramel, saffron, cardamom and cloves. Also works well with honey and dates.
Company%20profile
HAJJAN
It Was Just an Accident
Director: Jafar Panahi
Stars: Vahid Mobasseri, Mariam Afshari, Ebrahim Azizi, Hadis Pakbaten, Majid Panahi, Mohamad Ali Elyasmehr
Rating: 4/5
Groom and Two Brides
Director: Elie Semaan
Starring: Abdullah Boushehri, Laila Abdallah, Lulwa Almulla
Rating: 3/5